RESEARCH ARTICLE
Prevalence of Obstructive Sleep Apnoea Syndrome (OSAS) and Its Association with Hypertension in Jatinangor West Java
Badai B. Tiksnadi1, Arief Taufiqurrohman1, Agung D. Permana2, Faris Y. Fihaya3, *, Yulia Sofiatin4, Kurnia Wahyudi4, M. Rizki Akbar1, Rully M.A. Roesli5
Article Information
Identifiers and Pagination:
Year: 2019Volume: 11
First Page: 11
Last Page: 18
Publisher Id: TOHYPERJ-11-11
DOI: 10.2174/1876526201911010011
Article History:
Received Date: 10/07/2019Revision Received Date: 15/8/2019
Acceptance Date: 20/09/2019
Electronic publication date: 15/11/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Hypertension is a global health problem, with the prevalence increasing by 30% from 2013 to 2018 in Indonesia. Furthermore, obesity, a major risk factor for hypertension, has also escalated by 50%. Hence, the incidence of Obstructive Sleep Apnoea Syndrome (OSAS), which is strongly associated with hypertension and obesity, is expected to increase. OSAS is part of the complex sleep disorder breathing syndrome, but there is a lack of data regarding its prevalence and association with hypertension.
Objective:
To investigate the prevalence of OSAS and its association with hypertension in Jatinangor, West Java, Indonesia.
Methods:
A cross-sectional study was conducted from September to October 2018 of subjects from two villages in Indonesia selected by purposive sampling. Fifteen neighbourhoods were chosen by the cluster random sampling method, with a total of 1,308 respondents included in this study. Inclusion criteria were age > 17 years old and resident in the village for more than one year. OSAS was determined by a 4-variable screening tool questionnaire (4-V) and hypertension was measured by a standardised method (average of three measurements in each session with a one-minute break using a digital device); both measurements were performed by trained health cadres. All results were statistically analysed using chi-square and logistic regression.
Results:
Of the total of 1308 respondents included in this study, 33 (2.5%) had OSAS and 299 respondents (22.8%) had hypertension. In the population with OSAS, 18 respondents (54.5%) had hypertension, significantly higher (p<0.001) compared to the non-OSAS group (22%). After adjustment for age, gender, and Body Mass Index (BMI), OSAS was still an independent predictor of hypertension (OR = 4.3, p = 0.000).
Conclusion:
The prevalence of OSAS in the Jatinangor district of Indonesia is 2.5% and it is significantly associated with hypertension.
1. INTRODUCTION
Hypertension is the most common cause of cardiovascular disorders, therefore a major problem in developed and developing countries. Based on WHO data in 2015, around 1.13 billion people in the world suffer from hypertension. In addition, the prevalence of hypertension in Indonesia increased from 2013 to 2018, from 25.8% to 34.1% [1]. This is of great concern as effective blood pressure control is still suboptimal, even in high-income countries [2]. The incidence of factors associated with hypertension, such as obesity and obstructive sleep apnoea syndrome (OSAS), add further complexity to this issue.
Obesity represents a major health problem as it predisposes to cardiovascular disease, the leading cause of death globally every year [3]. Obesity prevalence in Indonesia increasing, almost 50% from 2013 to 2018, from 14.8 to 21.8% respectively. As obesity is probably the most important factor of OSAS, it may also lead to greater underdiagnosed prevalence of OSAS in Indonesia [4].
Secondary hypertension occurs in a significant proportion of adults with OSAS [5]. OSAS is part of the complex sleep disorder breathing syndrome and often occurs in adults. It is characterised by repeated episodes of upper airway obstruction during sleep, which causes breathing to stop partially (hypopnoea) or complete (apnoea). The overall estimated prevalence of OSAS ranges from 2-7% in adults [6], but the OSAS prevalence in Indonesia is not known.
OSAS escalates the incidence of cardiovascular disease five-fold regardless of age, smoking habits, weight or blood pressure [7, 8]. Moreover, untreated OSAS may contribute to underlying the origin and/or development of hypertension [9]. There is a complex mechanism of hypertension in OSAS involving various factors, such as sympathetic tone, peripheral vasoconstriction, endothelial dysfunction, increased Renin-Angiotensin-Aldosterone System (RAAS) activity and altered baroreceptor reflexes [10-12].
Based on previous population-based studies, the prevalence of hypertension increased by 1.42 to 1.72 times in moderate-severe OSA than non-OSA [13, 14]. Although having a complex mechanism, studies proved that endothelial dysfunction in OSAS patients could be restored with appropriate treatment, such as Continuous Positive Airway Pressure (CPAP) [15]. Nevertheless, data regarding the prevalence of OSAS and its relationship with hypertension in West Java, Indonesia is important but limited. Some studies have investigated the relationship between hypertension and OSAS in Indonesia, but none were population-based. Therefore this study aimed to investigate the prevalence of OSAS and its association with hypertension in Jatinangor District, West Java, Indonesia.
2. MATERIALS AND METHODS
2.1. Study Design
This study was an analytical study with a cross-sectional design to investigate the prevalence of OSAS and its association with hypertension in Jatinangor (West Java, Indonesia). The study was conducted from September to October 2018.
2.2. Sampling Method
Study participants were enrolled from two guided villages of Universitas Padjadjaran in Jatinangor using a multistage random sampling method (Fig. 1). Two selected villages (Cipacing and Cilayung village) of 12 villages in Jatinangor were chosen by a purposive sampling approach. Cipacing villages had 21 neighbourhoods and Cilayung village had ten neighbourhoods, so cluster random sampling was used to select six neighbourhoods of Cilayung village and nine neighbourhoods of Cipacing village. The inclusion criteria included:
(i) Age > 17 years old
(ii) Lived in the Cipacing or Cilayung for more than 1 year
Subjects were excluded from the study if they had at least one of the following exclusion criteria:
(i) Data of the subject in questionnaire was incomplete
(ii) Immigrant college student and/or worker
2.3. Procedure and Instrument
OSAS assessment used a 4-variable screening tool questionnaire (4-V), which has higher specificity than other instruments [16]. The cut-off point used in the 4-V was 14 according to the recommendation of Takegami et al. [17] The scoring formula was (gender*4) + (Body Mass Index (BMI) category value) + (Blood Pressure (BP) category value) + (snoring*4). Gender was set at 1 for males and 0 for females; BMI (< 21.0, 21.0-22.9, 23.0-24.9, 25.0- 26.9, 27.0-29.9, ≥ 30) was assigned a value between 1 and 6; BP (Systolic Blood Pressure (SBP) < 140 or Diastolic Blood Pressure (DBP) < 90, SBP 140-159 or DBP 90-99, SBP 160-179, or DBP 100-109, SBP ≥ 180 or DBP ≥ 110) was assigned a value between 1 and 4; and snoring was assigned 1 for a response of snoring almost every day or often, and 0 for snoring sometimes, almost never, or unknown.
Hypertension assessment was via the standard method BP measurement using a digital tension measurement device (ABN-100). Subjects were suggested to avoid drinking, smoking, coffee, tea and exercise for at least 30 minutes prior to the measurement. BP measurements were taken after the subjects rested in a seated position quietly for at least 10 minutes. The average of three measurements with a time interval of 1-2 minute between each measurement was recorded as the subject’s BP. Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. BMI index was according to Asian population criteria, with a normal BMI range between 18.5-22.9 kg/m2, overweight or at risk range between 23-24.9 kg/m2, and obese criteria is BMI ≥ 25 kg/m2.
This study involved 45 trained health cadres to conduct the OSAS and BP measurements. The health cadres were trained by a doctor twice in one month before collecting data. Collected data was also checked by a field assistant researcher until the data were complete.
2.4. Statistical Analysis
Statistical analysis was performed using SPSS Statistics 25. Sociodemographic data are presented as frequencies and proportions. Numerical data are presented as means and standard deviation. Chi-square test was used in the bivariate analysis of subjects’ characteristics (age, sex, BMI, and OSAS). In the bivariate analysis, Prevalence Ratio (PR), 95% Confidence Intervals (CI), and p-value for each independent variable were calculated, with a p-value < 0.05 indicating statistical significance, which then continued to multivariate logistic regression analysis.
3. RESULTS
In total, 1308 respondents were included in this study, consisting of 839 females (64.1%) and 469 males (35.9%). According to (Table 1), the mean age was 42.7 ± 15.33 and mean SBP and DBP were 126 ± 19.92 and 79.1 ± 10.49, respectively. Mean BMI was 23.8 ± 4.32, with 54% of subjects categorised as overweight or obese and the prevalence of hypertension was 22.8%. The respondents were divided into at risk-group (≥45 years) and not at risk-group (<45 years) according to age (Table 2), with the at risk-group being statistically significant for hypertension (p<0.001). Likewise, gender was associated with hypertension, with a significant (p<0.008) relationship between female respondents and hypertension. According to BMI, the overweight to obese group were more likely to have hypertension (p <0.001). Regarding OSAS variables, 33 respondents (2.5%) and 18 respondents (54.5%) in the OSAS group had hypertension. Non-OSAS group who had hypertension was 281 respondents (22%).
Based on the bivariate analysis (Table 2), the relationship of risk factors with hypertension from the strongest to the weakest was age (OR = 4.3), OSAS (OR = 4.24), BMI (2.70) and sex (OR = 0.68). Furthermore, the multivariable logistic regression analysis (Table 3) showed that age at risk (≥ 45 years), female, overweight to obese and OSAS were significantly associated with hypertension.
4. DISCUSSION
To the best of the authors’ knowledge, this is the first population-based study to investigate the prevalence of OSAS and its association with hypertension in Jatinangor, West Java, Indonesia. Based on the findings from 1,308 participants, the occurrence of hypertension was 22.8%, lower than the national prevalence of 34.1% [1]. There was an unequal gender proportion in this study and most participants were younger than <45 years, which may account for less hypertension than the national population. Furthermore, in a previous descriptive cross-sectional study in Jatinangor assessing 828 respondents, the prevalence of hypertension was 37.8%. Most hypertensive respondents in the previous study were female aged older than 70 years old [18], whereas the mean population age in the present study was only 42.7 years old.
OSAS prevalence was 2.5%, similar to the OSAS prevalence in a population-based study of a Persian population with 3,259 participants, 4.98% of which were high risk for OSAS [20]. This difference might be associated with the different tool used to assess the risk of OSAS. The 4-variable screening tool used in this study has high specificity, which may cause missed cases of OSAS, while the previous study used the berlin questionnaire with lower specificity [19]. Although the number of respondents at risk for OSAS tended to be lower than the previous study, more than 50% of those 33 OSAS respondents in this study had hypertension and respondents with OSAS had 4.2 times higher odds of having hypertension. This finding is higher than the previous study by Hedner et al, which showed that 46% of hypertensive respondents had OSAS [20]. The previous study used gold standard polysomnography to diagnose OSAS, while our study used screening tools to screen OSAS in the general population, which may account for the higher OSAS prevalence in our study [21]. Nonetheless, this is in line with a systematic review and meta-analysis conducted by Haifeng et al. (2018) of 51,623 respondents regarding the relationship of OSAS with hypertension, which found that OSAS was associated with the incidence of resistant hypertension [22]. Therefore, this finding supports the hypothesis of OSAS as a risk factor for hypertension.
Further multivariable logistic regression analysis showed various risk factors associated with hypertension. Age at risk (≥ 45 years), female, overweight to obese and OSAS were significantly associated with hypertension, with OSAS and age contributing more to hypertension, higher than BMI and gender. All variables were in accordance with a previous study which analysed phenotypic characteristic in 152 subjects with an equal proportion of hypertensive and normotensive participants [23]. However, the result regarding gender may be contradictory as male subjects are well known to have a higher risk of hypertension. A previous study to evaluate hypertension prevalence in Jatinangor involving 828 subjects showed the highest rate of hypertension in females aged >70 years [18]. Another study of 1,166 subjects with OSAS by Quintanna-Galego et al, stated that women were older, more obese and had hypertension more frequently in comparison to men [24], which indicates that women with a sleep disorder have a higher risk of hypertension than men.
OSAS is a clear risk factor for resistant hypertension. There is complex mechanism of hypertension in OSAS which involves various factors, such as sympathetic tone, peripheral vasoconstriction, endothelial dysfunction, increased RAAS activity and altered baroreceptor reflexes as shown in Fig. (2). Intermittent hypoxemia in apnoeic conditions increases Reactive Oxygen Species (ROS) and inflammatory cytokines. Predominantly, ROS are generated by mitochondrial dysfunction and increment of several enzymatic systems, such as xanthine oxidase, Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-oxidase, and NOS uncoupling, then react with Nitric Oxide (NO) resulting in diminished NO availability in the circulation [25]; this imbalance of vasoactive substances stimulates endothelial damage, dysfunction and vasoconstriction [10].
| Variable | n (%) | Mean ± SD |
|---|---|---|
| Age, years | - | 42.7 ± 15.33 |
| Sex | - | - |
| Female | 839 (64.1) | - |
| Male | 469 (35.9) | - |
| Blood Pressure | - | - |
| Systolic, mmHg | - | 126 ± 19.92 |
| Diastolic, mmHg | - | 79.1 ± 10.49 |
| BMI, kg/m2 | - | 23.8 ± 4.32 |
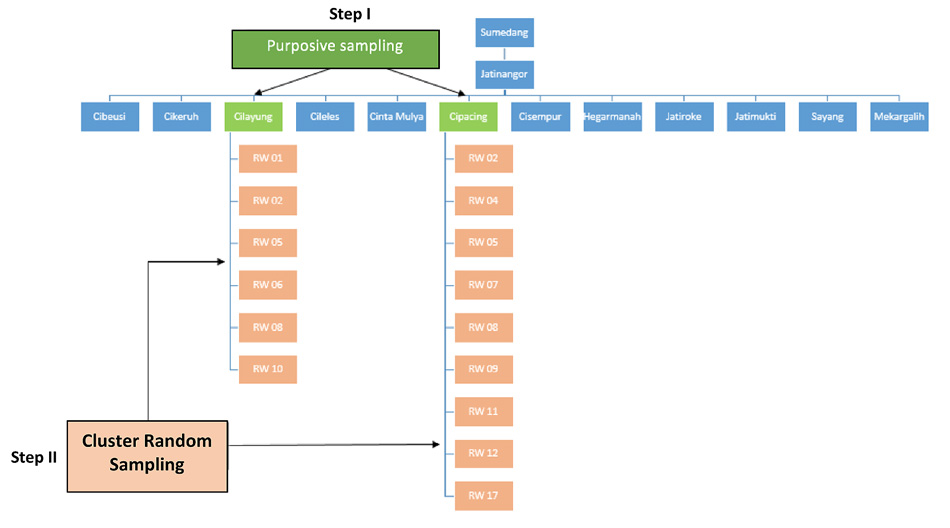 |
Fig. (1). Sampling method. RW: Rukun Warga (neighbourhood). |
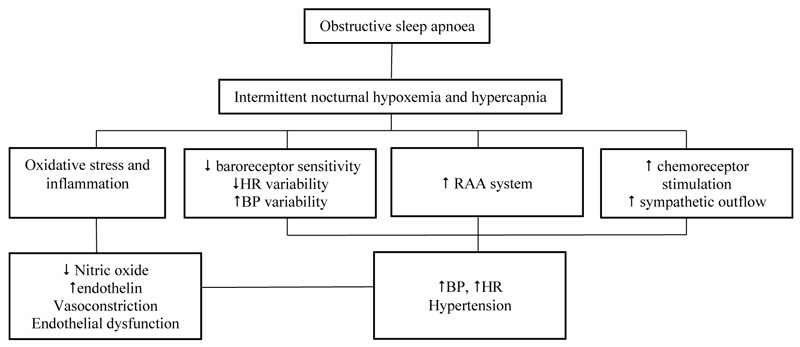 |
Fig. (2). Mechanism of hypertension and end organ damage in obstructive sleep apnoea. RAA: renin-angiotensin-aldosterone. [10, 11]. |
| Variable | Hypertension | Non-Hypertension | Total (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Subject (n) | 299 | 1009 | 1308 (100) | - | - | - |
| Age | - | - | - | 4.30 | 3.24-5.72 | <0.001 |
| At risk (<45 y) |
217 | 384 | 601 (45.9) | - | - | - |
| Non-risk (≥45 y) |
82 | 625 | 707 (54.1) | - | - | - |
| Sex | - | - | - | 0.68 | 0.52-0.90 | 0.008 |
| Female | 211 | 628 | 839 (64.1) | - | - | - |
| Male | 88 | 381 | 469 (35.9) | - | - | - |
| BMI | - | - | - | 2.70 | 2.03-3.58 | <0.001 |
| Overweight to Obese | 216 | 495 | 711 (54.4) | - | - | - |
| Normal | 83 | 514 | 597 (45.6) | - | - | - |
| OSAS | - | - | - | 4.24 | 2.11-8.53 | <0.001 |
| OSAS | 18 | 15 | 33 (2.5) | - | - | - |
| Non-OSAS | 281 | 994 | 1275 (97.5) | - | - | - |
| Variable | Hypertension | ||
|---|---|---|---|
| Adjusted β | 95% CI | P-value | |
| Age (At risk/Non-risk) | 4.33 | 3.23-5.80 | <0.001 |
| Sex (Female/Male) | 0.61 | 0.44-0.84 | 0.003 |
| BMI (Overweight to obese/Obese) | 2.32 | 1.72-3.14 | <0.001 |
| OSAS (Yes/No) | 4.24 | 1.93-9.27 | <0.001 |
Intermittent hypoxemia also stimulates carotid body receptors, causing catecholamine surges resulting in increased sympathetic response. Hypoxemia and hypercapnia also activate chemoreceptors which increase the sympathetic response and serum catecholamine. Impairment of baroreceptor reflexes in OSAS, blunted HR variability and increased BP variability are also typical in OSAS patients [10, 11]. In addition, RAAS activity also potentially contributes to hypertension, as OSAS is associated with higher angiotensin II and aldosterone levels [12], as well as increased blood pressure and pulse frequency followed by the start of normal ventilation after apnoea episodes. Hypoxia and hypercapnia are stimulated by the incidence of apnoea via autonomic nerves [25, 26].
Based on the review conducted in 2016 Figs. (4-8) [27], it was concluded that OSAS has the potential to be a risk factor for cardiovascular disease, as evidenced by the disruption of heart function and other cardiovascular diseases, such as coronary heart disease, atrial fibrillation and heart failure. A proposed diagnostic approach algorithm of OSAS associated with hypertension is shown in Fig. (3).
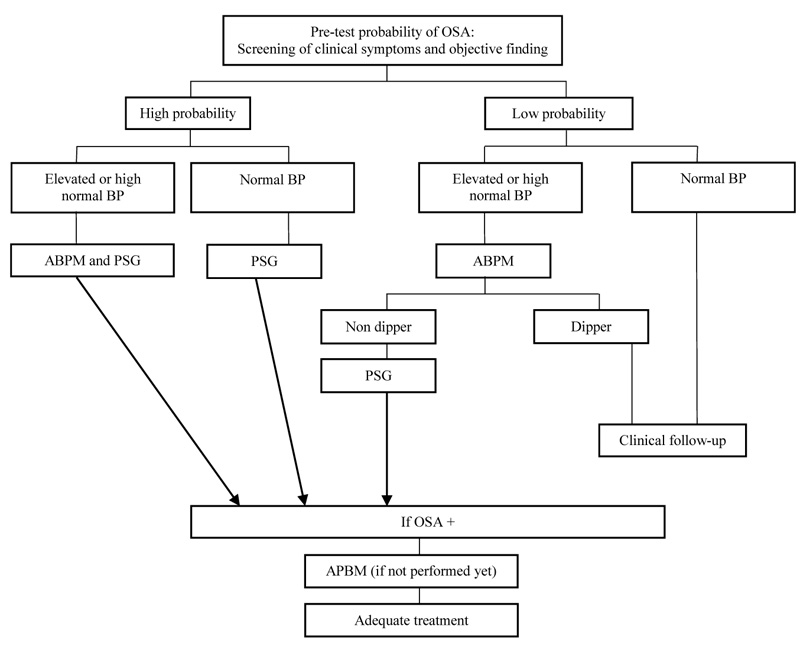 |
Fig. (3). Algorithm for a diagnostic and management approach of patients with hypertension associated with OSAS. ABPM: Ambulatory blood pressure monitoring; PSG: Polysomnography; BP: Blood pressure. [29]. |
 |
Fig. (4). Area of the study. |
CPAP is the main therapeutic device to correct the hypoxia related to sleep disorders, such as OSAS. There is evidence that OSAS treatments significantly reduce cardiovascular disease risk, therefore OSAS in resistant hypertensive patients should be the target of aggressive treatment [28]. Currently, management of OSAS and associated hypertension involves lifestyle changes, such as weight loss, reduced alcohol intake and regular exercise [29]. Systemic blood pressure could be ameliorated with effective CPAP treatment in moderate to severe OSAS, which then made the causal link between OSAS and hypertension clear [30]. Administering CPAP therapy for more than three months can improve the reduction in the risk of cardiovascular disease [16]. Medication history of the subject for hypertension and OSAS were not included in the study, but there is limited evidence to suggest that antihypertensive drugs would affect OSAS differently [31, 32].
Comorbidities, such as diabetes mellitus, the strongest predictor for hypertension in a previous study [33], were not assessed in the present study. However, based on a previous population-based study of 2,682 participants who were screened for the risk of OSAS, multivariate analysis was performed on 2.8% of the population who had OSAS, revealing that age, male gender, obesity and hypertension were independent risk factors for OSAS, but not diabetes mellitus [34]. Aside from obesity, middle-older aged individuals, and gender as factors contributing to the relationship of OSAS and hypertension were included in this study, but other factors such as alcohol intake and smoking were not evaluated in this study [35]. Other secondary causes of hypertension, such as primary aldosteronism and chronic kidney disease were also not evaluated in this study. The time of BP measurement is also important, with night-time BP being the best overall predictor for CV, and ambulatory BP was superior than clinic BP in our study [36]. Due to the limited risk factors evaluated in this study, the results should be considered with caution.
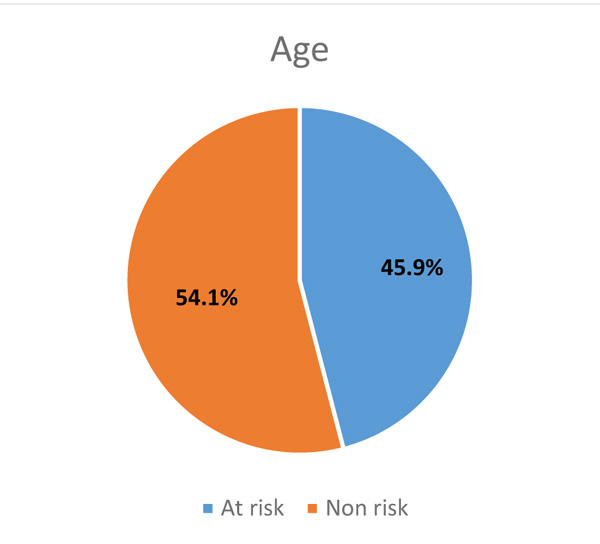 |
Fig. (5). Prevalence of population based on age. |
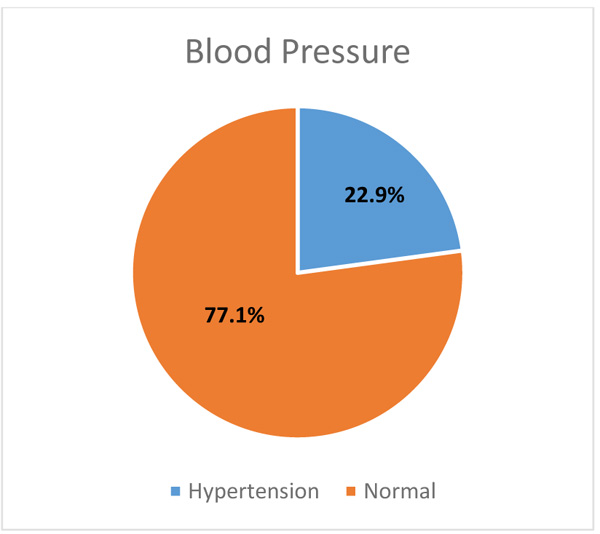 |
Fig. (6). Prevalence of population based on blood pressure. |
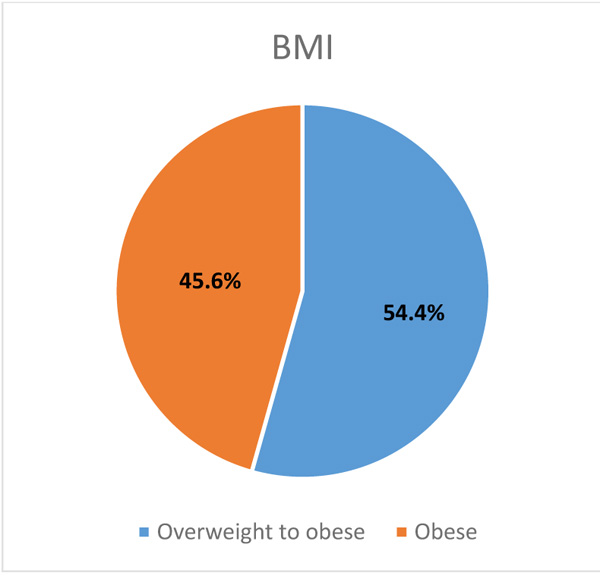 |
Fig. (7). Prevalence of population based on BMI. |
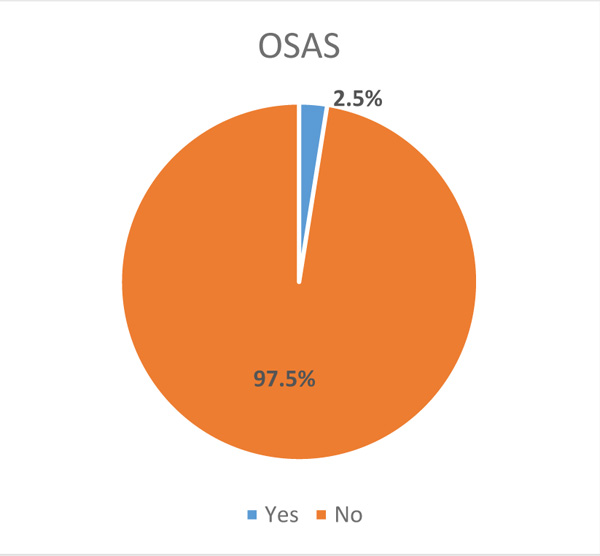 |
Fig. (8). Prevalence of population based on OSAS. |
CONCLUSION
The prevalence of OSAS in a sample of the population in the Jatinangor District in Indonesia is 2.5% and is associated with hypertension.
LIMITATIONS
The instrument used in this study was a questionnaire to determine OSAS, which may have information bias. Despite the high specificity of the 4-V tool in determining OSAS, it has relatively low sensitivity compared to other tools, which could lead to missed cases of OSAS [17]. In addition, diabetes and other factors contributing to hypertension, such like alcohol intake, smoking, hyperaldosteronism and chronic kidney disease were not assessed in this study. Blood pressure measurements were not validated, however they were performed by trained non-physicians, therefore missed or over-diagnosed hypertension may have occurred.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was issued by the Health Research Ethics, Faculty of Medicine, Padjadjaran University (No. 27/UN6. KEP/EC/2018).
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Informed consent has been obtained from all the participants.
STANDARD OF REPORTING
STROBE Guideline and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed throughout this research are included in this published article.
FUNDING
This study received internal grant from Padjadjaran University, Indonesia. (No.2476/UN6.C/LY/208)
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We appreciate the assistance of health cadres in Cipacing and Cilayung Village.
REFERENCES
| [1] | Kementrian Kesehatan RI. Hasil utama Riskesdas 2018 Jakarta: Kemenkes 2018. Available at: http://www.depkes.go.id/resources/ download/info-terkini/materi_rakorpop_2018/Hasil%20Riskesdas% 202018.pdf |
| [2] | Bloch MJ. Worldwide prevalence of hypertension exceeds 1.3 billion. J Am Soc Hypertens 2016; 10(10): 753-4. |
| [3] | Kyrou I, Randeva HS, Tsigos C, et al. Clinical Problems Caused by Obesity [Updated 2018 Jan 11] In: Feingold KR, Anawalt B, Boyce A, et al, editors Endotext [Internet] South Dartmouth (MA): MDTextcom, Inc https://www.ncbi.nlm.nih.gov/books/NBK278973/ |
| [4] | Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension 2003; 42(6): 1067-74. |
| [5] | Puar THK, Mok Y, Debajyoti R, Khoo J, How CH, Ng AK. Secondary hypertension in adults. Singapore Med J 2016; 57(5): 228-32. |
| [6] | Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5(2): 136-43. |
| [7] | Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med 2002; 165(9): 1217-39. https://www.ncbi.nlm.nih.gov/pubmed/ 11991871 |
| [8] | Welch KC, Goldberg AN. Sleep disorders.Current diagnosis & treatment, otolaryngology head and neck Surgery 2nd ed. 2008; 535-47. |
| [9] | Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010; 7(12): 677-85. |
| [10] | Ahmad M, Makati D, Akbar S. Review of and updates on hypertension in obstructive sleep apnea. Int J Hypertens 2017; 13. |
| [11] | Phillips CL, O’Driscoll DM. Hypertension and obstructive sleep apnea. Nat Sci Sleep 2013; 5: 43-52. |
| [12] | Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol 2016; 13(4): 333-43. |
| [13] | Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med 2000; 160(15): 2289-95. |
| [14] | Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283(14): 1829-36. |
| [15] | Ciccone MM, Favale S, Scicchitano P, et al. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. Int J Cardiol 2012; 158(3): 383-6. |
| [16] | Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: Comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med 2011; 7(5): 467-72. |
| [17] | Takegami M, Hayashino Y, Chin K, et al. Simple four-variable screening tool for identification of patients with sleep-disordered breathing. Sleep 2009; 32(7): 939-48. Available at:
https://www.ncbi.nlm.nih.gov /pmc/articles/PMC2706898/ |
| [18] | Fihaya FY, Sofiatin Y, Ong PA, Sukandar H, Roesli RMA. Prevalence of hypertension and its complications in Jatinangor 2014. J Hypertens 2014; 33e35 |
| [19] | Pataka A, Daskalopoulou E, Kalamaras G, Fekete Passa K, Argyropoulou P. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med 2014; 15(7): 776-81. |
| [20] | Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med 1985; 103(2): 190-5. |
| [21] | Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: A population-based case-control study. Eur Respir J 2006; 27(3): 564-70. |
| [22] | Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J Glob Health 2018; 8(1)010405https://www.ncbi.nlm.nih.gov/pmc /articles/PMC5825975/ |
| [23] | Drager LF, Pereira AC, Barreto-Filho JA, et al. Phenotypic characteristics associated with hypertension in patients with obstructive sleep apnea. J Hum Hypertens 2006; 20(7): 523-8. |
| [24] | Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: A clinical study of 1166 patients. Respir Med 2004; 98(10): 984-9. |
| [25] | Lombardi C, Parati G, Cortelli P, et al. Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res 2008; 17(3): 263-70.https://onlinelibrary.wiley.com/doi/full /10.1111/j.1365-2869.2008.00659.x |
| [26] | Noda A, Nakata S, Koike Y, et al. Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndrome. Hypertens Res 2007; 30(8): 669-76.https://www.nature .com/articles/hr200791 |
| [27] | Maeder MT, Schoch OD, Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc Health Risk Manag 2016; 12(1): 85-103.https://www.ncbi.nlm.nih.gov /pmc/articles/PMC4807890/ |
| [28] | Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 2007; 176(12): 1274-80. |
| [29] | Parati G, Lombardi C, Hedner J, et al. EU COST Action B26 members. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J 2013; 41(3): 523-38. |
| [30] | Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1): 68-73. |
| [31] | Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5): 1423-8. |
| [32] | Furlan SF, Braz CV, Lorenzi-Filho G, Drager LF. Management of hypertension in obstructive sleep apnea. Curr Cardiol Rep 2015; 17(12): 108. |
| [33] | Bawazir LA, Sianipar WPH. Determinants of blood pressure control and prevalence of hypertension in adults in 2017: A population-based study in West Jakarta. Open Hypertens J 2018; 10: 15-27. |
| [34] | Wali SO, Abalkhail B, Krayem A. Prevalence and risk factors of obstructive sleep apnea syndrome in a Saudi Arabian population. Ann Thorac Med 2017; 12(2): 88-94. |
| [35] | Dorasamy P. Obstructive sleep apnea and cardiovascular risk. Ther Clin Risk Manag 2007; 3(6): 1105-11. |
| [36] | Kario K, Kanegae H, Tomitani N, et al. Nighttime blood pressure measured by home blood pressure monitoring as an independent predictor of cardiovascular events in general practice. Hypertension 2019; 73(6): 1240-8. |




